http://ei.cornell.edu/toxicology/bioassays/lettuce/default.html
The idea behind a reference toxicity test is that the test
organism, in this case lettuce seeds, will respond in a predictable
manner to varying concentrations of a particular chemical compound.
At some threshold concentration, all of the test organisms will
be killed (or in this case, none of the lettuce seeds will sprout).
In solutions that are more dilute, some level of inhibition will
occur in seed germination and/or radicle length. If the concentration
is low enough, no response will be detectable.
This is called a dose/response experiment. You vary
the dose of a selected compound, then measure the response of the bioassay
organism.
Using NaCl In order to determine
whether lettuce seeds provide a good bioassay for salt toxicity, you can conduct
a reference test using known concentrations of NaCl (table salt).
First, make a 0.2M NaCl solution by mixing 11.69 g NaCl
with enough deionized water to make 1 liter.
Second, label a series of beakers with the following concentrations:
0.2M, 0.1M, 0.075M, 0.05M, and 0.025M. Make up these concentrations from the
0.02M solution using the proportions listed in the following table:
| Concentration
|
0.2 M NaCl (mL)
|
Deionized Water (mL)
|
| 0.2 M NaCl
|
100.0
|
0
|
| 0.1 M NaCl
|
50.0
|
50.0
|
| 0.075 M NaCl
|
37.5
|
62.5
|
| 0.050 M NaCl
|
25.0
|
75.0
|
| 0.025 M NaCl
|
12.5
|
87.5
|
| Control
|
0
|
100.0
|
1. Treat the lettuce seeds in a 10% bleach solution for 20
minutes, then rinse five times with deionized or distilled water.
This kills fungal spores that can interfere with seed germination.
Note: Tap water can be used if you do not have access to deionized
or distilled water, but it will introduce more variability into
your experiment because of the varied minerals and other compounds
it contains.
2. In each of 18 9-cm petri dishes, place a 7.5-cm paper filter.
Label the dishes according to the first column in the following
table. Note: Absorbent paper towels or coffee filters can
be substituted for the filter paper, as long as they are first
shown to be be nontoxic. (Bleached paper may contain dyes or
chlorine.)
3. To each petri dish, add 2 ml of the appropriate test solution.
In the control dishes, use deionized water as your test solution.
4. To each dish, add 5 lettuce seeds, spaced evenly on the
filter paper so that they do not touch each other or the sides
of the dish.
5. Place the dishes in a plastic bag and seal it to retain
moisture. Incubate the seeds in the dark at constant temperature
(preferably 24.5 degrees C) for 5 days (120 hours).
6. At the end of this time, count how many seeds in each dish
have germinated, and measure the root length
of each to the nearest mm. Look carefully at the plants to make
sure you are measuring just the root, not the shoot as well.
Using Other
Compounds
To be useful, a bioassay must be sensitive to the types of
compound you are interested in evaluating. For example, if you
are worried about herbicide contamination of ponds or streams,
a bioassay based on seed germination might prove to be more sensitive
than one based on death of fish or invertebrates. On the other
hand, fish are likely to be much more sensitive than seeds to
a compound that is a nerve toxin, for example.
To determine the sensitivity of an organism to a chemical
compound, scientists carry out reference toxicity tests. To do this, you measure
the response of the organism to a wide range of concentrations of the selected
chemical. What concentrations should you use? That of course depends on both
the bioassay organism and the chemical being tested. You might want to start
by searching through
published
student reports included on this web site to see whether anyone else has
already generated data that would be of use to you.
Before scientists begin an experiment, usually they search
through published scientific literature for papers that relate
to the procedure they have in mind. If you have access to scientific
journals, it would be a good idea to look for papers that report
bioassays using the organism and compound you are interested
in (see
References for example
papers). This is a good way to get an idea about an appropriate
range of concentrations.
If you can't find any appropriate data, that's ok -- you'll
just have to start with a broader range of concentrations to
make sure you hit the range that your test organism responds
to. (With too high a concentration, the test organisms will all
die, or in the case of seeds, none will sprout. With too low
a concentration, you will not be able to detect any difference
between your samples and your control.) Ideally, you want to
test concentrations that cover both of these endpoints plus a
range of concentrations in between. Then you will be able to
conclude whether your test organism responds in a predictable
way to the compound you are testing.
Serial dilutions are one way to set up a broad range of concentrations.
For example, suppose you suspect that in a 100 mg/L solution
of a selected compound, no lettuce seeds will sprout, and you
are interested in narrowing this down to find out the range of
concentrations in which germination will occur. You might decide
to start with a 10-fold dilution series, testing solutions of
100, 10, 1, 0.1 and 0.01 mg/L. Another possibilitity would be
a dilution series in which each solution is half the strength
of the previous solution in the series: 100, 50, 25, 12.5, and
6.25 mg/L.
Once you have collected data using an initial set of concentrations,
you may find that it would be useful to carry out a follow-up
experiment using a more narrow set of concentrations. For example,
if none of the seeds sprout at one concentration in your series,
and all of them sprout at the next level of dilution, it would
make sense to carry out a dilution series between these two concentrations
in order to further define the sensitivity of lettuce seeds to
your selected compound.
Taking Measurements At the
end of the 5-day growth period, count and record how many seeds in each dish
have germinated.
For each sprout, measure the radicle length to the nearest
mm. (The radicle is the embryonic root).
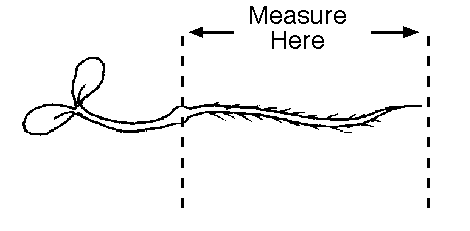
Look carefully at the plants to make sure you are measuring
just the radicle, not the shoot as well. For example, in the
picture below, you would measure just the part between the two
arrows, not the shoot and cotelydons to the left.
How Good are Your Data?
Once you have counted how many seeds germinated, and measured
their radicle lengths, then what? How can you interpret these
results?
Comparison to the Control
The first thing to check is your control (the dishes that
contain deionized or distilled water rather than a sample). The
purpose of the control is to identify how well the seeds will
grow without any added contaminants. Would you expect all of
the seeds in your control dishes to germinate? Probably not,
just like a gardener does not expect all the seeds in a garden
to sprout.
If fewer than 80% of the seeds in your control dishes sprouted,
something may have gone wrong in your experiment. Perhaps the seeds were too
old or stored improperly, so they were no longer viable. Or maybe something
went wrong with the conditions for growth. Did the dishes get too hot, too
dry, or contaminated in some way? Did you use tap water for your control, rather
than deionized or distilled water? In many cases this works fine, but since
tap water is highly variable from source to source, it gives less predictable
results.
A Look at VariabilityWithin each treatment, how much
variability did you find in your results? Did the replicate dishes show similar
numbers of seeds sprouting, and similar average radicle lengths? If you think
the data are much more variable than you would expect, you might want to explore
the potential
sources of variability
for this type of experiment.



































